Protein Blobs Linked to Alzheimer’s Affect Aging in All Cells
This is an information dense article that may be of interest to Steve, and others, given we all age. I have pulled out some summary points below.
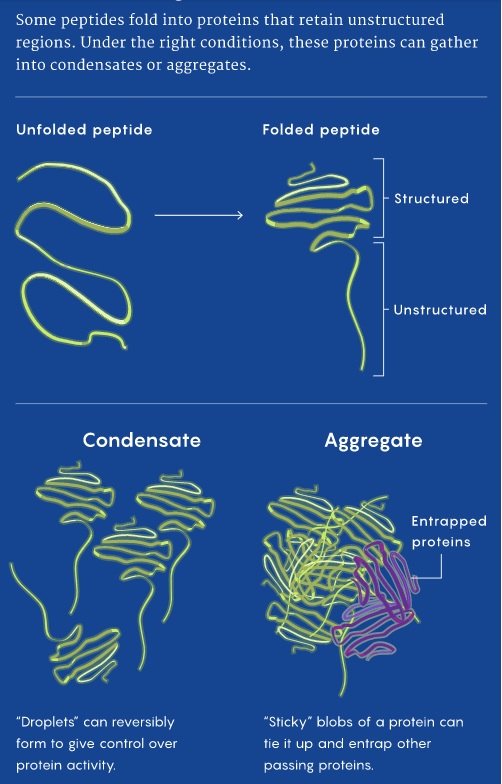
a recent study by a team of Stanford University researchers suggests that protein aggregation may be a universal phenomenon in aging cells and could be involved in many more diseases of aging than was suspected.
The study showed that protein aggregation probably contributes to the gradual deterioration of many tissues over time. The findings even offer a hint about why these aggregates are so much more obvious in the brain than in other tissues: It may be because brains have been evolving so rapidly.
a huge mystery in human biology is why these neurodegenerative diseases are so tissue specific,” said Cynthia Kenyon. No one really knows, for example, why the amyloid protein plaques of Alzheimer’s disease form in the hippocampus of the brain and the aggregates in Parkinson’s disease are specific to dopamine neurons.
proteins that aggregate with age and that can actually catalyze further aggregation of proteins in a prionlike manner, which was not shown before,” he said.
If the amyloid plaques characteristic of the disease form to protectively bind up the defective protein, then breaking up the plaques might do more harm than good.
“It is a hard concept for humans to grasp, since it seems intuitive that things that look abnormal should be ‘bad’ and pathogenic,” Finkbeiner wrote. “But biology is complex, full of many feedback loops,
The picture emerging clearly now is that protein aggregation isn’t a phenomenon restricted to neurodegenerative diseases: It is part of every cell that lives long enough to age.
And the fact that protein aggregation throughout the body is a factor in the aging of organisms as disparate as yeast, worms, flies, fish, mice and humans, she added, “means that we, as a field, should be paying a lot more attention to this.”
Thanks _ I missed this.